Usher Syndrome Research
Finding treatments and a cure is not a matter of if, but when
The Usher Syndrome Society is dedicated to finding treatments and a cure for all types of Usher syndrome. To date, we have committed over $3.5 Million to research efforts in labs around the world focused on advancing critical hearing and vision research related to Usher syndrome.
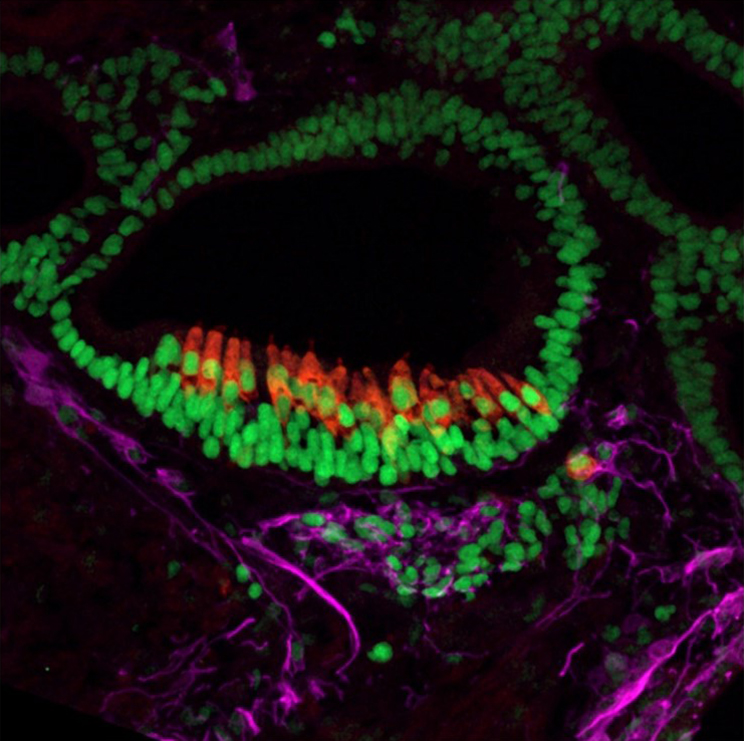
Microscopic Image of a 55-day old inner ear organoid
Pipeline for Usher Syndrome Research
The Pipeline for Usher Syndrome Research (PUSH) was launched January 2025. This groundbreaking project aims to tackle multiple forms of Usher Syndrome in parallel, identifying subtypes and mutations that may be amenable to innovative therapies.
By advancing potential therapies over the next three years, PUSH will strive to identify optimized strategies that can progress toward clinical trials, ultimately improving treatment options for vision and hearing loss associated with USH.
Pipeline for Usher Syndrome Research (PUSH)
The Pipeline for Usher Syndrome Research (PUSH) is a groundbreaking project at Boston Children’s Hospital, launched with an initial large gift from the Usher Syndrome Society, to accelerate treatment development for Usher Syndrome (USH). This innovative project brings together six world-class scientists in one academic institution, each a leader in their field with complementary expertise. PUSH is uniquely designed to address multiple forms of Usher Syndrome in parallel, identifying specific subtypes and mutations that may respond to cutting-edge therapies. While labs worldwide are working on USH treatments, PUSH is the first collaborative effort of this scale, aiming to establish a new pipeline to speed the translation of therapies to clinical application for various types of Usher Syndrome.

Microscopic Image of a 55-day old inner ear organoid
USS Translational Research Grants
In 2022, The Usher Syndrome Society established our Scientific Advisory Committee and founded the USS Translational Research Grants. These grants are intended to support translational research on Usher syndrome in either Preclinical Research and/or Mechanism-based Therapeutic Development.
The research projects include well-documented research collaborations across sensory modalities and across scientific disciplines. All approaches are collaborative, designed to target cells in the eye and the ear.
Please contact us to inquire about our Usher Syndrome Society Translational Research Grant applications and eligibility.

2025 Grant Recipients
GRANT RECIPIENT #1:
Monte Westerfield, University of Oregon
A screen for compounds that prevent or slow retinal, auditory, and vestibular cell death in Usher syndrome.
Dr. Monte Westerfield and his lab are conducting preclinical studies to identify compounds that can be used as therapeutics for treatment of vision, hearing, and balance problems in most, if not all forms of USH. This includes Types 1F, 1B, 1C, 1D, 1G, 2A, 2C, 2D, 3A.
They chose promising compounds reported to have neuroprotective effects on retinal cells and are testing this panel for efficacy in treating zebrafish models of Usher syndrome. Monte and his team systematically treat zebrafish Usher gene mutants with each compound and assess photoreceptor and inner ear hair cell death and visual, auditory, and vestibular function. Compounds that provide significant improvement become candidates for clinical trials.
GRANT RECIPIENT #2:
Vasiliki Kalatzis, Vision Team at the Institute for Neurosciences of Montpellier (INM) in Montpellier, France
Developing a large vehicle for gene replacement therapy of Usher syndrome.
Usher syndrome is caused by changes (called mutations) in certain genes that affect how cells in the eye and ear work. One possible treatment is to add a healthy copy of the faulty gene into the diseased cells. However, some Usher genes are so large that they don’t fit into the currently used vehicles, creating a major obstacle for therapy.
Dr. Vasiliki’s project aims to overcome this challenge by testing a new, larger delivery vehicle to carry the biggest Usher gene (USH2A) into diseased eye cells. As a first step, they already showed that this new vehicle can successfully deliver a trackable gene into healthy human “mini-retinas” – small lab-grown retinal structures. They have also made mini-retinas from the skin cells of people with Usher syndrome who carry mutations in the USH2A gene, and have found differences between these diseased mini-retinas compared to healthy ones.
The next step of their project is to put the large USH2A gene into the new vehicle, deliver it into the diseased mini-retinas, and see if it can help restore the cells to a healthier state. If successful, this vehicle could also be used to treat other large Usher genes.
GRANT RECIPIENT #3:
Merel Stemerdink Radboudumc in Nijmegen
Advancing preclinical evaluation of therapeutic strategies using patient-derived 3D retinal organoids for ADGRV1-associated retinitis pigmentosa
On April 1, 2025, the follow-up research on Usher syndrome type 2C (USH2C), began led by Merel Stemerdink within the research group of Dr. Erwin van Wyk at the Radboudumc in Nijmegen, focusing on developing an innovative model to test new therapies.
The Usher Syndrome Society and Stichting Ushersyndroom jointly funded the first year of the project, after which the LifeLong Vision program will support the subsequent two years of research. Thanks to the valuable contributions from Swedish and American families, who raised funds through various initiatives and events, the USH2C research can now take an important next step.
The goal of this research is to develop an organoid model – a three-dimensional cell system that mimics the various cell types of the retina. This model is derived from patient cells, ensuring that the genetic background matches that of the patient. This gives the organoid model a key advantage over the animal models currently in use, such as zebrafish, as it enables therapy testing within the patient’s own genetic context.
The Radboudumc in Nijmegen is already working on the development of therapies for USH2C. Initial tests in zebrafish have shown promising results. The next step is to test these therapies in the newly developed USH2C organoids, which are now being produced.
Previous Grant Recipients
2024
Piloting Preclinical Development of Patient-Customized ASO Therapies for USH 2A
Piloting Preclinical Development of Patient-Customized ASO Therapies for USH 1B
These projects are headed up by the collaborative team of Dr. Tim Yu and Dr. Gwen Géléoc at Boston Children’s Hospital and Harvard Medical School and Dr. Monte Westerfield at University of Oregon. They are developing novel antisense oligonucleotides (ASOs for short) that target mutations in exons 6, 19, 20 and a mutation that leads to a pseudo-exon formation between exon 40 and 41 of the USH2A gene. They are using the same approach to also target a founder mutation in USH1B. The ASO strategy uses short stretches of RNA designed to selectively block USH mutations and allow for generation of healthy USH proteins. In this case, Drs. Yu and Geleoc plan to test the approach in cell lines, in Zebrafish, and in inner ear and retinal organoids derived from Human stem cells. If successful, this ASO approach may alleviate some of the consequences of these USH2A and USH1B mutations.
2024
Westerfield Drug Screening
The project, led by Dr. Monte Westerfield, is focused on the evaluation of experimental drugs and drugs already approved by the FDA for their ability to alleviate the symptoms of Usher syndrome. The work is being done in zebrafish that carry genetic mutations in Usher syndrome genes. The zebrafish is a good animal model for this work because the fish grow quickly and are inexpensive, which means Dr. Westerfield and his team can screen a lot of different drugs in many different zebrafish models of USH which will include USH1F, USH1B, USH1C, USH1D, USH1G, USH2A, USH2C, and USH3A. In addition, at the cellular and genetic level, the inner ears and retinas of zebrafish are quite similar to those of humans. Thus, discoveries in zebrafish may be more easily translated for use in humans.
2022-2023
Rescuing The Common Deep Intronic USH2A Variant c.7595- 2144A>G by innovative EDCas9 Genome Editing
This project that the Usher Syndrome Society funded is being done in cell lines that carry an intronic mutation in the USH2A gene. This project, led by Suzanne Kohl in Germany, is examining a part of the USH2a gene that does not code for protein but is important for how the protein is assembled. The mutation leads to the formation of truncated non-functional USH2A protein. Dr. Kohl’s team is developing a new CRISPR method for genome editing of the USH2A mutation. The goal is to disrupt the mutation, allowing for the formation of a healthy, fully-functional form of the USH2A protein.
Dr. Vasiliki Kalatzis, PhD
Vasiliki Kalatzis, PhD, is a molecular biologist with a long-standing research interest in genetic disorders. She performed her undergraduate studies in genetics and microbiology atthe University of Adelaide in Australia. She then moved to Paris, France, in 1993 to perform her Ph.D. training at the Pasteur Institute, where she identified the gene responsible for Branchio-Oto-Renal syndrome. In 1998, she moved to the Necker Hospital in Paris as a post-doctoral fellow to study the lysosomal storage disease Cystinosis and, in 2001, as a tenuredInserm scientist, she moved to the Institute of Molecular Genetics in Montpellier, to develop gene therapy as a potential treatment. During this time she became interested in the retina, and, in 2010, she joined the INM to study inherited retinal diseases (IRDs). Vasiliki Kalatzis is currently an Inserm research director and head of the Vision team at the INM. She is also a clinical interface researcher of the Montpellier University Hospital affiliated with the national reference centre for inherited sensory disorders, Maolya.
The main focus of the Vision team is to develop patient-specific induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium and sensory organoid models for diverse IRDs to elucidate genetic variants, decipher pathophysiological pathways and develop novel therapies (pharmacological, gene supplementation and genome editing).

Merel Stemerdink
Merel started as a PhD candidate in 2020 in the Usher Syndrome Therapy Group, where she focused on developing genetic therapies for Usher syndrome type 2C (USH2C), one of the subtypes of Usher syndrome that is caused by mutations in the ADGRV1 gene. Two therapeutic approaches have been explored as potential future treatments:
- Minigene Augmentation Therapy – Developing miniaturized versions of the ADGRV1 gene that encode for smaller versions of the ADGRV1 protein with sufficient residual function, to compensate for the defective ADGRV1 gene in patients.
- Exon Skipping Therapy – Bypassing the mutated sections (exons) of the ADGRV1 gene, allowing the body to produce a slightly shorter but still functional protein.
To evaluate these approaches, a zebrafish model was generated with targeted mutations in the adgrv1 gene, mimicking the retinal dysfunction seen in USH2C patients. While evaluation of the minigene strategy is ongoing, her work already successfully demonstrated that skipping of well-selected exons (= exon-skipping) can restore retinal function in the zebrafish model. This represents an important milestone, as it marks the first potential therapeutic intervention strategy for ADGRV1-associated vision loss.
Following the completion of her PhD project, she is now developing retinal organoids—miniature, lab-grown retinas derived from USH2C patient cells. This represents an important step forward, as these organoids provide a platform to evaluate therapeutic strategies in a human, patient-specific genetic context—an essential next step in the preclinical development of treatments for USH2C.

Dr. Erwin van Wijk
Dr. Erwin van Wijk is an Associate Professor at the Department of Otorhinolaryngology at Radboudumc in Nijmegen, The Netherlands. He leads a research team focused on the development of novel splice modulation and gene augmentation therapies for Usher syndrome, employing zebrafish as an in vivo model system. His team obtained pre-clinical therapeutic proof of concept for multiple USH2A targets, one of which has progressed to a Phase 1/2 clinical trial (#NCT03780257) with promising results, leading to the recent initiation of a Phase 2b trial (#NCT06627179). Currently, his group is also working on the phenotypic analysis and development of innovative treatments for other types of Usher syndrome.

Dr. Gwen Géléoc
Dr. Géléoc is an Associate Professor in the Department of Otolaryngology and the Kirby Neurobiology Center at Boston Children’s Hospital. The primary goals of her research is to characterize the functional and molecular development of the sensory cells in the inner ear and to use this knowledge to develop novel therapies for the treatment of hearing and balance disorders, in particular those associated with Usher syndrome. She earned a PhD in France and did post-doctoral work at University College London and Mass General Hospital.

Dr. Timothy Yu
Dr. Yu is an Associate Professor in the Division of Genetics & Genomics at Boston Children’s Hospital. Dr. Yu’s research is focused on the intersection of genomics, informatics, and neurobiology to better understand and treat rare neurologic diseases. Research efforts span the range from gene discovery for autism and other neurodevelopmental disorders to novel approaches for advancing individualized genomic medicine, including rare genetic disorders such as Usher Syndrome. A graduate of Harvard College, Dr. Yu completed MD-PhD training at UC San Francisco and neurology residency at Massachusetts General Hospital and Brigham and Women’s Hospital.

Dr. Monte Westerfield
In his lab that studies Usher syndrome, Dr. Monte Westerfield’s group showed that endoplasmic reticulum stress is the proximal cause of sensory cell death in Usher syndrome, which helped to discover the first known genetic modifier, PDZD7. Dr. Westerfield directs the Zebrafish Core of the Model Organism Screening Center for the Undiagnosed Diseases Network of the NIH. He received his AB degree in physics and biology from Princeton University and his PhD from Duke University Medical School. His postdoctoral training was completed at the Max Planck Institute in Munich, Germany and at Harvard Medical School before he joined the faculty in the Institute of Neuroscience at the University of Oregon. There, he met George Streisinger and began using zebrafish in his research. He established and currently directs ZFIN, the Zebrafish Information Network, and ZIRC, the Zebrafish International Resource Center.


Dr. Suzanne Kohl
Dr. Kohl studied Biology at the University of Tübingen and received her diploma in Human Genetics, Zoophysiology and Biochemistry in 1996 and her PhD in 2000. During her PhD, she identified the first gene for Achromatopsia and over the years described all other genes that are causative for this rare disease. Dr. Kohl has authored over 160 peer-reviewed manuscripts and is actively involved in grant and manuscript reviews. She has spent her entire scientific career at the University Eye Hospital and Institute for Ophthalmic Research in Tübingen, and her research focuses on the molecular genetics and functional analysis of genes and proteins involved in inherited retinal diseases (IRD). Her personal goal is to provide a genetic diagnosis to each individual IRD patient, thereby confirming the clinical diagnosis, providing information on inheritance and recurrence risk, and enabling patients to participate in natural history studies and clinical trials, and ultimately to receive gene- or mutation-specific therapy.
Pietro De Angeli M.Sc.
Mr. De Angeli received his master’s degree in Molecular Biotechnology at the University of Urbino, defending a thesis on “High-throughput mutational profiling of Cas9 endonucleases”. As a part of the European training network StarT, he has conducted his Ph.D. research at the Centre for Ophthalmology, University Hospital Tübingen, focusing on the establishment of “CRISPR/Cas9-based strategies to rescue deep-intronic variants in ABCA4”. His long-term scientific goal is to bring safe and effective therapies to patients with IRDs, therefore, he is now committed to investigate innovative genome editing approaches allowing permanent correction of genetic defects for Usher syndrome and Retinitis pigmentosa.

additional Research we support
Research is happening all over the world for Usher syndrome. Although funding for a rare disease is difficult, we will continue to support as many scientists as we can until we find a cure. We are always looking for additional opportunities to collaborate with research teams and fund novel research projects. If you would like your work or the work of a known research team considered for funding by the Usher Syndrome Society, please contact us.

Massachusetts Eye and Ear
The Usher Syndrome Society supports the Ocular Genomics Institute at Mass Eye and Ear directed by Dr. Eric Pierce. Dr. Qin Liu, an investigator at the Institute, has a research team that is focused on developing genome editing technologies for the treatment of retinal degeneration due to mutations in a number of genes, including USH2A. Results from these studies continue to be promising.
Holt/Géléoc Lab Boston Children’s Hospital
The Usher Syndrome Society has supported research in the Holt/Géléoc lab at Boston Children’s Hospital for many years. Noteworthy donations from the USH Society have funded an ABR machine and the development of a large animal model for USH2A to further characterize the pathophysiology of USH2A. This model generated excitement in the field and attracted the attention of industry partners. As a direct result of the seed funding provided by the Usher Syndrome Society, the Holt/Géléoc lab began a collaboration with an industry partner to develop novel therapeutics for Usher Syndrome. In addition, the Holt/Géléoc lab is actively developing therapeutics to target three other Usher Syndrome genes using a range of cutting-edge techniques.

Stephanie Mauriac, Ph.D.
Weill Cornell Medicine
With the help of Usher Syndrome Society donations, Dr, Ching-Hwa Sung, and her team are actively pursuing the mechanistic actions by which the diseased Müller cells affect visual functions and retinal health in the novel USH 3A mouse models. The lab is also investigating whether Müller cells produce other Usher Syndrome proteins.
University of Iowa Institute of Vision Research
Using generous support from the Usher Syndrome Society and other philanthropists, investigators of the Kimberling Usher Research Laboratory at the University of Iowa Institute for Vision Research (IVR) have:
-
- Devised a culture system for human donor retina to allow novel viral vectors to be developed for gene therapy of large Usher genes.
- Doubled the size of our gene therapy manufacturing facilities to allow multiple new treatments per year to be manufactured in a nonprofit setting.
- Developed a modular robot-assisted stem cell reprogramming and differentiation facility to speed our development of patient-derived polymer supported photoreceptor grafts.
- Continued Project Usher, a philanthropically supported program that allows individuals with Usher syndrome who cannot afford commercial genetic testing to obtain a state of the art genetic test.
- Embarked upon a natural history study of all forms of Usher syndrome. We already have data in hand from more than 250 patients with disease-causing genotypes in one of these genes

Dr. Ian Han & Dr. Edwin Stone
Help us find treatments and a cure.
Dr. Eric Pierce
Dr. Eric Pierce is a full-time clinician-scientist and Director of the Ocular Genomics Institute at Mass Eye and Ear, and the William F. Chatlos Professor of Ophthalmology at Harvard Medical School. As a group, inherited retinal degenerations (IRDs) are a common cause of blindness. The goals of Dr. Pierce’s clinical and lab-based research programs are to improve the understanding of the genetic causes of IRDs and to help develop genetically informed therapies for these diseases inclusive of all forms of Usher syndrome.

Dr. Ching Hwa Sung
Dr. Ching-Hwa Sung, Betty Neuwirth Lee and Chilly Professor in Stem Cell Research at Weill Cornell Medicine, employs state-of-the-art genetic manipulations to generate mice expressing the Usher disease mutant proteins. Her overarching goals are to develop interventions to halt the progression of retinal degeneration through a fundamental understanding of the pathological basis of these diseases.




